223
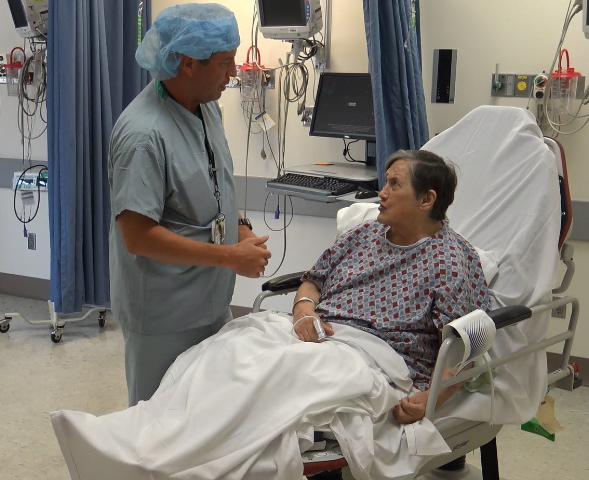
A Minden woman is the first patient at University Health in Shreveport to receive an ultra-small pacemaker without having to open her chest.
Juanita Bui, a resident at Meadowview Health and Rehab in Minden, underwent the procedure Tuesday to regul
World’s smallest pacemaker used in Minden woman
previous post